CanWell announced today that the preclinical research results and clinical plan of the company’s TLR7 agonist CAN1012 would be presented at the 4th STING & TLR Targeted Therapy Summit in 2023.
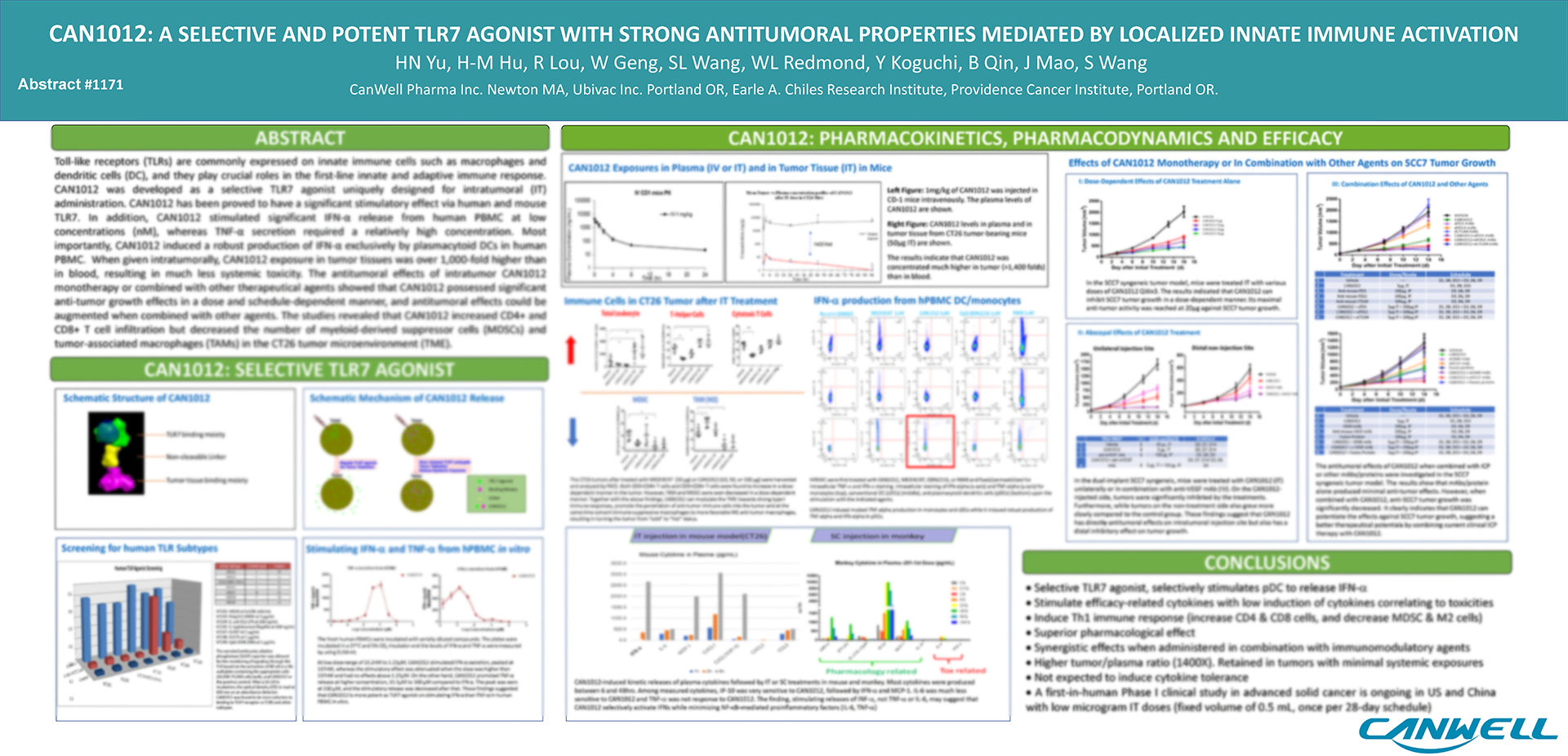
CAN1012 is a highly selective small-molecule TLR7 agonist developed by CanWell with global intellectual property rights. Preclinical studies have shown that it has high selectivity and potency, with low toxicities. It can exert sustained stimulation of the target cells in the tumor microenvironment, transforming “cold” tumors with low immune response into “hot” tumors with augmented immune response, playing a crucial bridging role in the innate and adaptive immune systems. In addition, it may be administered in combination with various drugs such as immunotherapy checkpoint inhibitors, as well as radiotherapy and chemotherapy, to produce highly synergistic effects.
The CAN1012 Phase I clinical trial is an open-label, multicenter, dose-escalation, and dose-expansion study aimed at evaluating the safety, tolerability, and preliminary efficacy of the drug in late-stage solid tumor patients as a single agent, and determining the maximum tolerated dose and recommended Phase II dose. Currently, the study is being conducted simultaneously in China and the United States.
The 4th STING & TLR Targeted Therapy Summit brought together more than 100 industry-leading immune targeted therapy organizations from the large biopharmaceutical industry and academic institutions. The event provides a unique opportunity to present and share the latest development and progress in the field of STING and TLR agonist based therapeutics.