GUANGZHOU, China – March 3, 2026 – CANWELL Phrama today announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to CAN1012, the company’s proprietary IFNα-biased TLR7 agonist, for the treatment of Soft Tissue Sarcoma (STS). This designation represents a significant milestone in CANWELL Phrama’s global strategic roadmap, validating the clinical potential of CAN1012 in addressing high unmet medical needs within the field of refractory oncology.
- About CAN1012
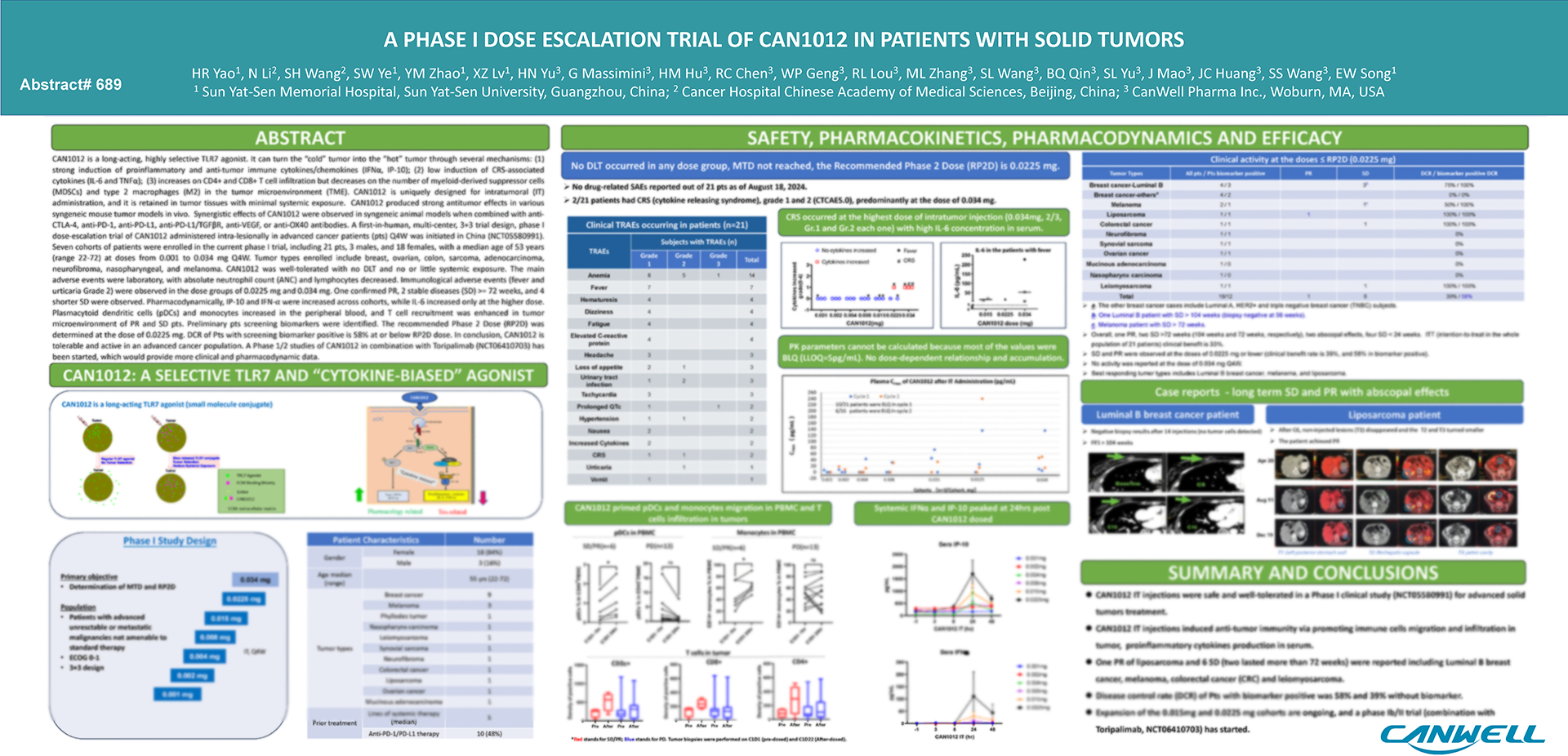
CAN1012 is a Class I innovative small-molecule Toll-like receptor 7 (TLR7) selective agonist independently developed by CANWELL Phrama with full global intellectual property rights. Distinguished as the only TLR7 agonist in its class globally capable of biased induction of IFNα, CAN1012 specifically recognizes TLR7 to trigger robust IFNα production while maintaining minimal pro-inflammatory cytokine responses (such as IL-6). This unique profile results in high efficacy paired with low toxicity. The candidate is currently in Phase II clinical development.
At the 2025 European Society for Medical Oncology (ESMO) Annual Meeting, CANWELL Phrama presented preliminary data from a Phase Ib/II trial evaluating CAN1012 in combination with toripalimab for advanced solid tumors:
- Dedifferentiated Liposarcoma (DDLPS): In evaluable patients, the combination therapy achieved an Objective Response Rate (ORR) of 25%, significantly outperforming the current standard-of-care chemotherapy (doxorubicin monotherapy ORR is typically ~2.9%). Given that DDLPS is traditionally insensitive to radiotherapy and chemotherapy, these results suggest CAN1012 could provide a breakthrough for this patient population.
- PD-1 Refractory Melanoma: In evaluable patients with advanced melanoma who progressed after PD-1 inhibitor therapy, the combination achieved an ORR of 37.5% and a Disease Control Rate (DCR) of 100%. This indicates CAN1012’s potential to reverse PD-1 resistance.
- Safety Profile: The incidence of Grade 3 Treatment-Related Adverse Events (TRAEs) was only 3.5%, with no reported Grade 4/5 TRAEs, Cytokine Release Syndrome (CRS), or Dose-Limiting Toxicities (DLTs).
- About Soft Tissue Sarcoma (STS)
Soft Tissue Sarcoma is a group of malignant tumors originating from connective tissues—including fat, muscle, nerves, and blood vessels—encompassing over 70 subtypes. According to the American Cancer Society, approximately 13,520 new cases and 5,410 deaths were projected in the U.S. in 2025. While STS accounts for 1% of adult malignancies, it represents up to 15% of pediatric cancers. Due to its high heterogeneity and risk of recurrence, patients with advanced STS face limited therapeutic options and poor prognoses.
The FDA Orphan Drug Designation will accelerate the clinical development and regulatory process of CAN1012 in the United States, offering renewed hope to patients worldwide.
- About FDA Orphan Drug Designation
The FDA’s Orphan Drug Act provides incentives to encourage the development of drugs for rare diseases affecting fewer than 200,000 people in the U.S. Benefits include federal tax credits for clinical research costs, waivers of PDUFA application fees, and seven years of market exclusivity upon regulatory approval.
About CANWELL Phrama
CANWELL Phrama is a clinical-stage biopharmaceutical company dedicated to developing First-in-Class (FIC) and Best-in-Class (BIC) cancer immunotherapies. Led by a founding team with extensive global R&D and executive experience at multinational pharmaceutical companies, the firm focuses on clinical value and patient outcomes.
CANWELL Phrama has established two core technology platforms: a Multi-payload ADC (Antibody-Drug Conjugate) platform and an SMDC (Small Molecule Drug Conjugate) platform. Leveraging these technologies, the company has built a robust pipeline of innovative immuno-oncology and targeted therapies, several of which have progressed into clinical trials.